MUSCLOR-oC
Skeletal muscle is the most abundant tissue in the human body and plays essential roles in locomotion, respiration, thermoregulation, and metabolic homeostasis. Its integrity is critically compromised in a wide range of conditions, from neuromuscular disorders such as Duchenne Muscular Dystrophy and Spinal Muscular Atrophy, to age-related sarcopenia and chronic diseases including cancer and diabetes. Despite major progress in disease modeling and regenerative therapies, current in vitro models of human skeletal muscle fail to reproduce the broad physiological complexity of native muscle tissue, particularly its spatial organization, cellular complexity and domain-specific functionality. There is an urgent need for human-relevant, physiologically faithful muscle systems to advance fundamental research, therapeutic screening, and personalized medicine.
Coordinator

University Professor and Hospital Partitioner
Inserm
Institutions and establishments involved
Work Packages
CNRS; Inserm; Université Paris-Est Créteil; Sorbonne Université; UGA; U Strasbourg
The consortium’s objectives are structured into four major Work Packages
- WP1 : Engineering a 3D innervated human muscle incorporating motor neurons and Schwann cells, to recreate functional NMJs.
- WP2 : Introduction of muscle connective tissue fibroblasts and tendon constructs into the system to form physiologically relevant MTJs and reconstitute the muscle-tendon continuum.
- WP3 : Integrating both interfaces in a unified platform, enabling precise spatial organization and microfluidic perfusion to mimic vascular supply.
- WP4 : Application of this system to model two representative frequent neuromuscular disorders — Duchenne Muscular Dystrophy and Spinal Muscular Atrophy — using patient-derived iPSCs and gene therapy paradigms, providing proof-of-concept for personalized therapeutic testing.
Research program
The MUSCLOR-oC project aims to develop a next-generation human skeletal muscle-on-chip platform that replicates not only the structural and contractile features of muscle fibers, but also integrates the two critical anatomical and functional interfaces: neuromuscular junctions (NMJs) and myotendinous junctions (MTJs). These are essential for muscle innervation and force transmission, respectively, and are commonly affected in muscular diseases. Existing systems lack the co-existence of both NMJ and MTJ within the same construct, limiting their translational relevance. Our project brings together a multidisciplinary consortium of experts in muscle biology, tissue engineering, organ-on-chip technologies, preclinical modeling, developmental biology and transcriptomic analysis
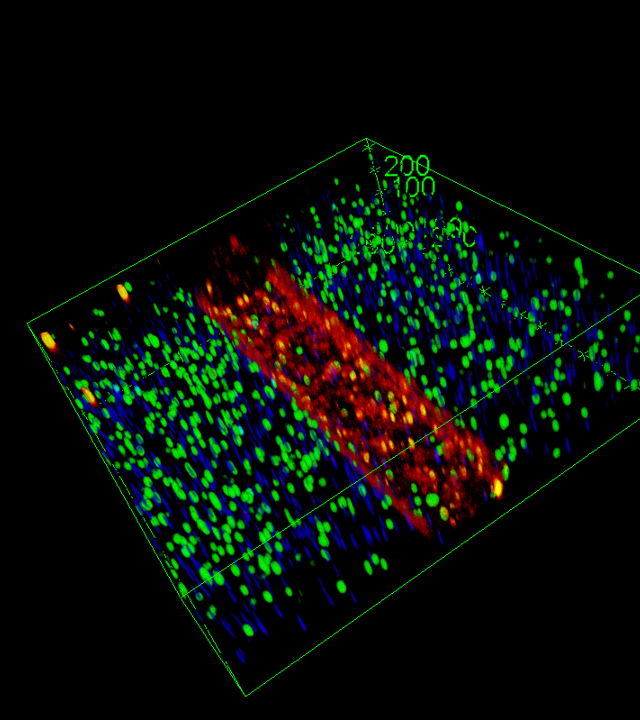
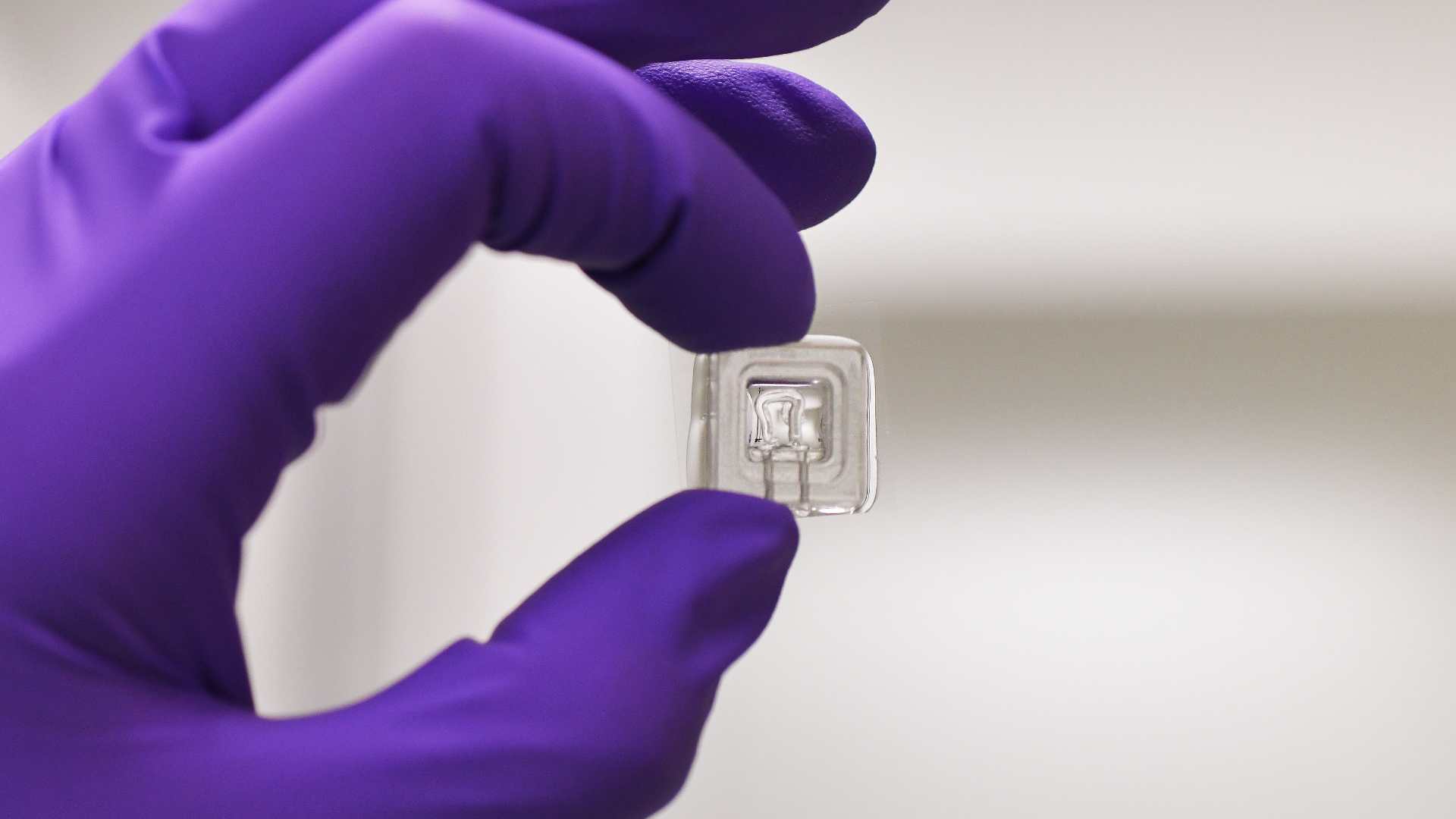
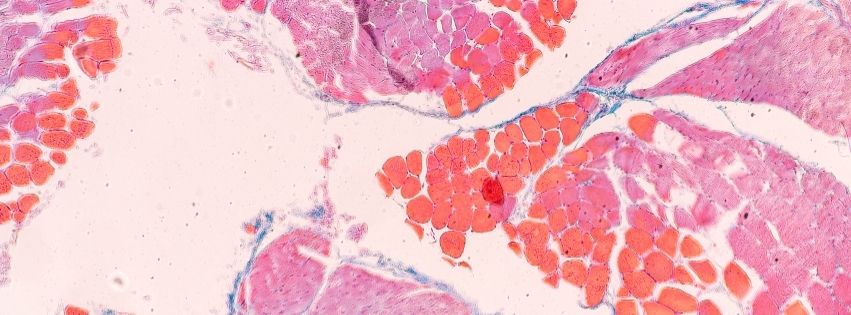
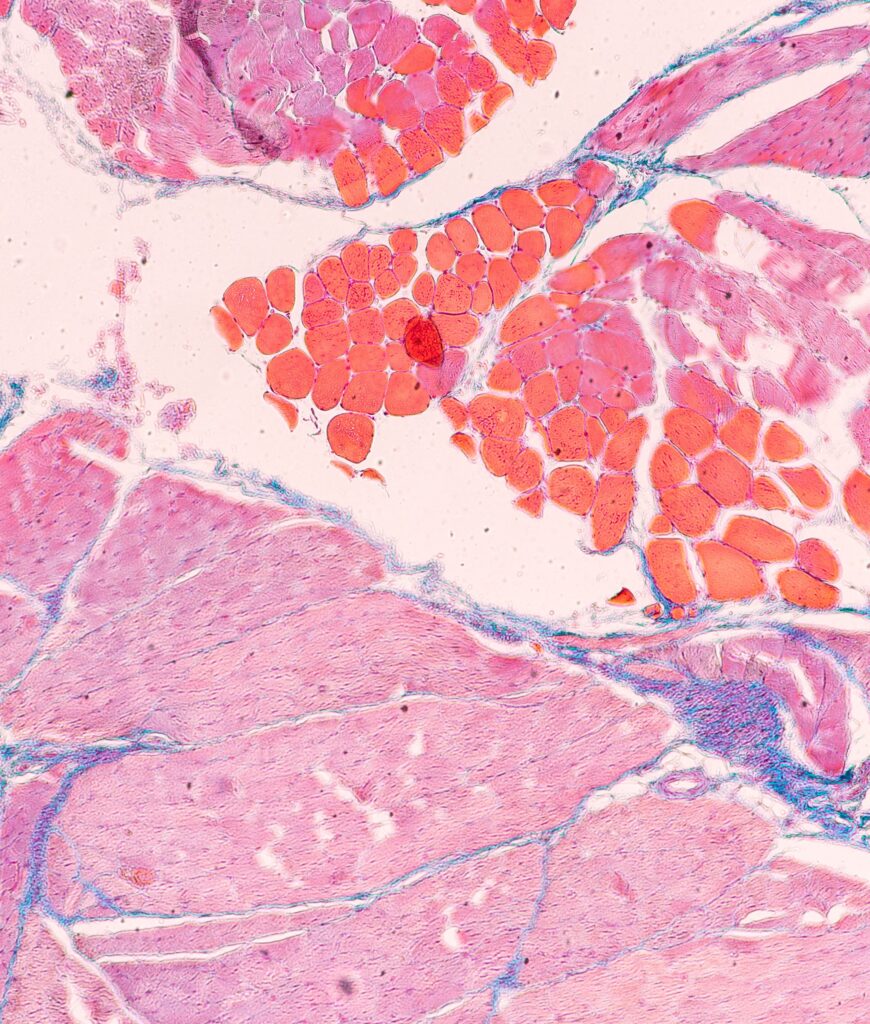
Preliminary data from the partners demonstrate the feasibility and performance of engineered 3D muscle tissues derived from both primary muscle stem cells and hiPSC sources. These constructs exhibit mature myofiber features, contractile activity, and pathological signatures that recapitulate disease phenotypes. Additional technological innovations include customized PDMS-based chips designed for modularity and real-time force readouts, as well as novel biomaterials that enhance myogenic differentiation and structural organization. Integration with cutting-edge single-nucleus RNA-sequencing and RNA-FISH approaches ensures molecular fidelity and enables high-resolution domain-specific analysis.

Expected outcomes
MUSCLOR-oC will deliver a transformative tool for the French and international research communities, bridging the gap between muscle biology, disease modeling, and translational medicine. The platform will be scalable, modular, and adaptable for high-throughput screening. It will also provide a foundation for building clinical twin models and stratifying patient responses to gene or drug therapies. The project is fully aligned with the goals of the PEPR “Organ On Chip” initiative, offering a unique contribution in the underexplored field of skeletal muscle systems. By targeting key unmet needs in neuromuscular research and integrating technological innovation with logical insight, MUSCLOR-oC holds strong potential to accelerate the development of next-generation therapies and improve patient outcomes.
The consortium
Our project brings together a multidisciplinary consortium of experts in muscle biology, tissue engineering, organ-on-chip technologies, preclinical modeling, developmental biology and transcriptomic analysis.
Institut Mondor de la Recherche Biomédicale (IMRB); Developmental Adaptation and Aging Laboratory (Dev2A); Laboratoire Interdisciplinaire de Physique (LIPhy); Institut de génétique et de biologie moléculaire et cellulaire (IGBMC)
Plus de projets