Multi-organ coupling on a chip for metabolic syndrome monitoring
(MSY-OOC)
Non-alcoholic fatty liver disease (NAFLD, now called MAFLD) is a growing public health problem, affecting 25% of European adults. 10–30% of cases progress to non-alcoholic steatohepatitis (NASH), which can lead to cirrhosis or hepatocellular carcinoma. MAFLD is closely linked to metabolic syndrome (MSy), a complex multi-organ disease associated with pathologies such as type 2 diabetes, obesity and cardiovascular diseases. In Europe, MSy affects up to 36% of the population.
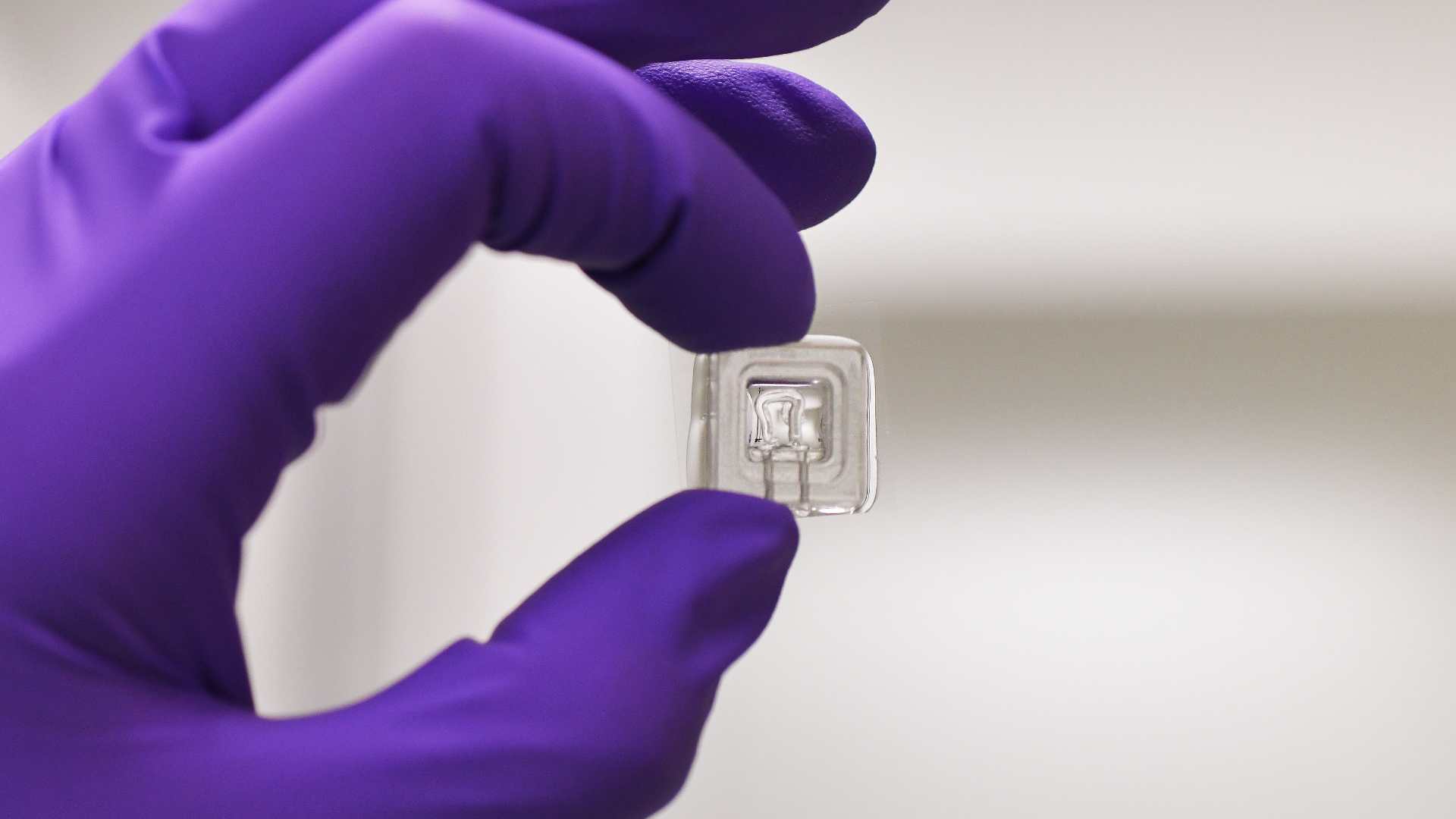
Understanding the mechanisms of disease progression is essential to improve risk stratification for patients and develop innovative treatments. Animal models are insufficient to reproduce human metabolism. Although liver-on-chips are already used for toxicology studies, these models do not fully reproduce the functional unit of the perfused human liver.
To propose and validate new in vitro models, the consortium, with 20 years of experience in liver engineering and microphysiological systems, draws on the international expertise of the Antoine-Béclère Hospital and the CHB, recognized for the management of serious liver disorders and for their translational research units in hepatology.
The goal is to develop a new generation of organs-on-a-chip capable of reproducing cellular interactions and the mechanical and chemical properties of the extracellular matrix (ECM) of the liver, in healthy and pathological conditions, as well as interactions with other organs involved in metabolic syndrome. This model will allow:
- To identify new biomarkers and evaluate innovative therapies using data from patient cohorts.
- To study the mechanisms of MAFLD onset: lipid accumulation, dysfunction of hepatic sinusoid endothelial cells (LSECs) and activation of stellate cells leading to fibrosis.
- To simulate metabolic syndrome (MSy): creation of a demonstrator reproducing the interaction between different organs involved in MSy (liver, adipose tissue, vascular wall), by integrating the effects of the environment or specific diets.
La stéatose hépatique non alcoolique (NAFLD, maintenant appelée MAFLD) est un problème de santé publique croissant, touchant 25 % des adultes européens. 10 à 30 % des cas évoluent vers une stéatohépatite non alcoolique (NASH), pouvant conduire à une cirrhose ou un carcinome hépatocellulaire. La MAFLD est étroitement liée au syndrome métabolique (MSy), une maladie complexe affectant plusieurs organes et associée à des pathologies telles que le diabète de type 2, l’obésité et les maladies cardiovasculaires. En Europe, le MSy touche jusqu’à 36 % de la population.
Comprendre les mécanismes de progression de ces maladies est essentiel pour améliorer la stratification des risques pour les patients et développer des traitements innovants. Les modèles animaux sont insuffisants pour reproduire le métabolisme humain. Bien que les « liver-on-chip » (foie sur puce) soient déjà utilisés pour des études de toxicologie, ces modèles ne reproduisent pas complètement l’unité fonctionnelle du foie humain perfusé.
Pour proposer et valider de nouveaux modèles in vitro, le consortium, fort de 20 ans d’expérience en ingénierie hépatique et en systèmes microphysiologiques, s’appuie sur l’expertise internationale de l’Hôpital Antoine-Béclère et du CHB, reconnus pour la gestion des troubles hépatiques graves et pour leurs unités de recherche translationnelle en hépatologie.
L’objectif est de développer une nouvelle génération d’organes sur puce capable de reproduire les interactions cellulaires et les propriétés mécaniques et chimiques de la matrice extracellulaire (ECM) du foie, en conditions saines et pathologiques, ainsi que les interactions avec d’autres organes impliqués dans le syndrome métabolique. Ce modèle permettra :
- D’identifier de nouveaux biomarqueurs et d’évaluer des thérapies innovantes grâce à des données issues de cohortes de patients.
- D’étudier les mécanismes d’apparition de la MAFLD : accumulation de lipides, dysfonctionnement des cellules endothéliales du sinusoïde hépatique (LSEC) et activation des cellules stellaires menant à la fibrose.
- De simuler le syndrome métabolique (MSy) : création d’un démonstrateur reproduisant l’interaction entre différents organes impliqués dans le MSy (foie, tissu adipeux, paroi vasculaire), en intégrant les effets de l’environnement ou de régimes alimentaires spécifiques.
Coordinators

CNRS Research Director
UMR BMBI (BioMechanics and
Bioengineering)
CNRS and Université de Technologie
de Compiègne (UTC)
CNRS

CNRS Research Director
IRL 2820 LIMMS
CNRS/U Tokyo
CNRS

PUPH in gastroenterology and hepatology
hepatology at the Centre Hépato-Billaire of Hôpital Paul-Brousse
APHP/INSERM/Université Paris Saclay
Inserm
Institutions and establishments involved
CNRS UMR 8520 IEMN et INSERM U1193 et U996, UMRS 1138 ; Hôpital Antoine Béclère
Research program
Reproducing on-chip the structure and functions of a steatotic liver/MAFLD:
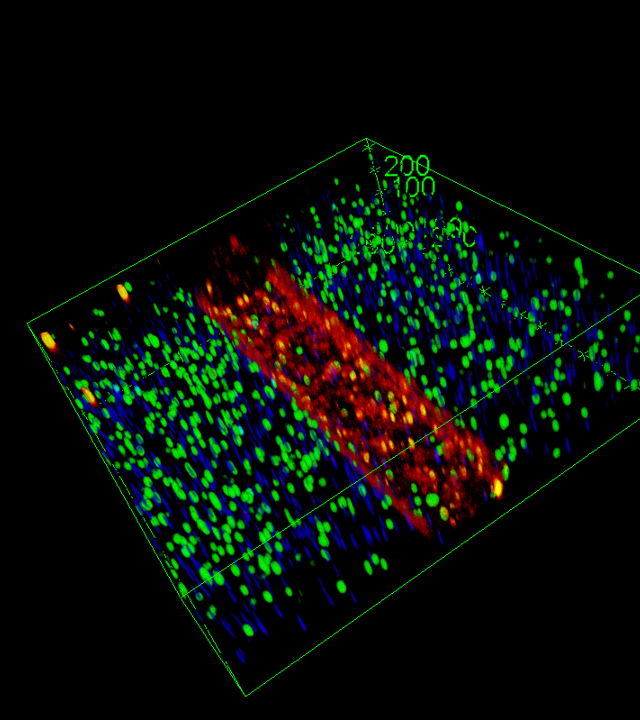
Development of a functional sinusoidal barrier by culturing LSECs on a soft matrix, then more rigid to simulate fibrosis.
Accumulation of toxic lipids in hepatocytes to reproduce lipotoxicity.
Co-culture with stellate cells or hepatic macrophages (Kupffer cells) to induce inflammation.
Validation of the model by comparing in vitro data with serological and histological data from patients with MAFLD.
Coupling the key organs of the metabolic syndrome (liver, adipose tissue, vascular wall):
Adaptation of perfusion flows and culture media to simulate physiological and pathological conditions
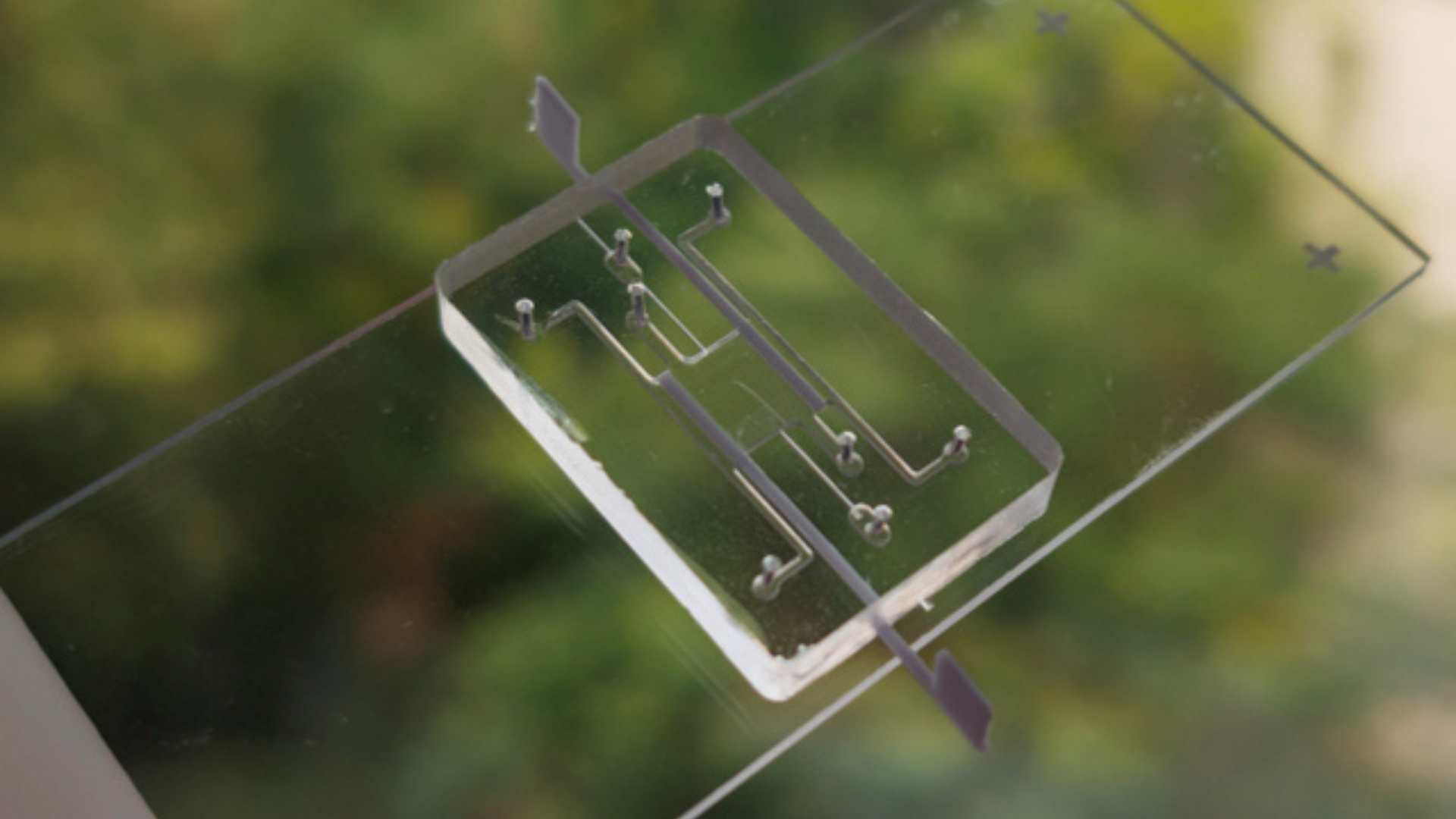
Use of the patented CCDIM Box platform, allowing to perfuse and couple several organs on a chip in series or in parallel.
Development of a new microfluidic device for multi-organ coupling
Identify biomarkers of MSy progression and evaluate treatments:
Exploitation of data from the LITONAS, ObPlus and PLICATURE cohorts (> 400 patients) with serum, liver and adipose tissue samples.
Validation of biomarkers such as miRNA-122/CK18 levels, pro-inflammatory cytokines, and expression of fibrotic proteins (α-SMA/Col1A1).
Integration of multi-omics data and imaging analyses into in silico models to identify new biomarkers and signaling mechanisms.
The Consortium
- UMR CNRS 7338 Biomécanique et Bioingénierie (BMBI) / UTC : C. Legallais, R. Jellali, M. Vayssade, , A. Le Goff, S. Morandat
- IRL CNRS 2820 LIMMS / U Tokyo : E. Leclerc, F. Soncin, Y. Sakai
- UMR CNRS 8520 IEMN : A. Treizebré
- (UMRS iCAN : F. Foufelle
- CHB/APHP/INSERM/Université Paris Saclay : J-C. Duclos Vallée
- Hôpital Antoine Béclère/APHP/INSERM U996 : C. Voican
Plus de projets