MOHICAN
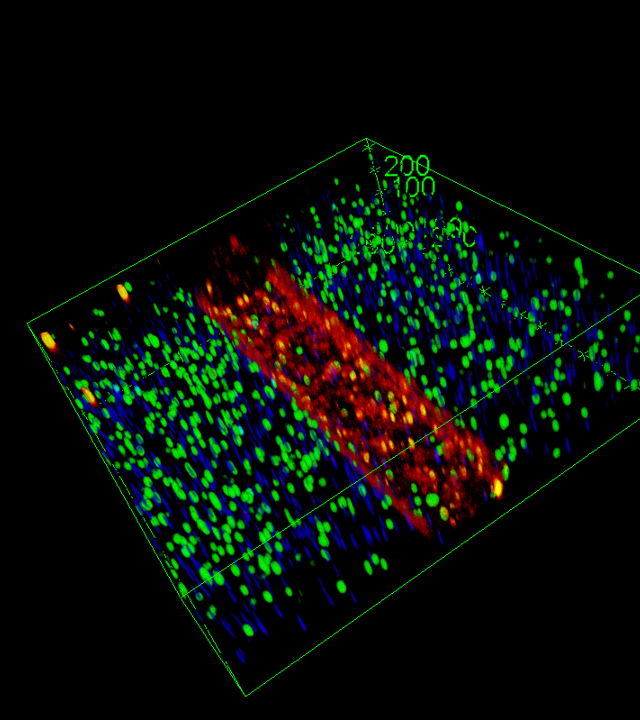
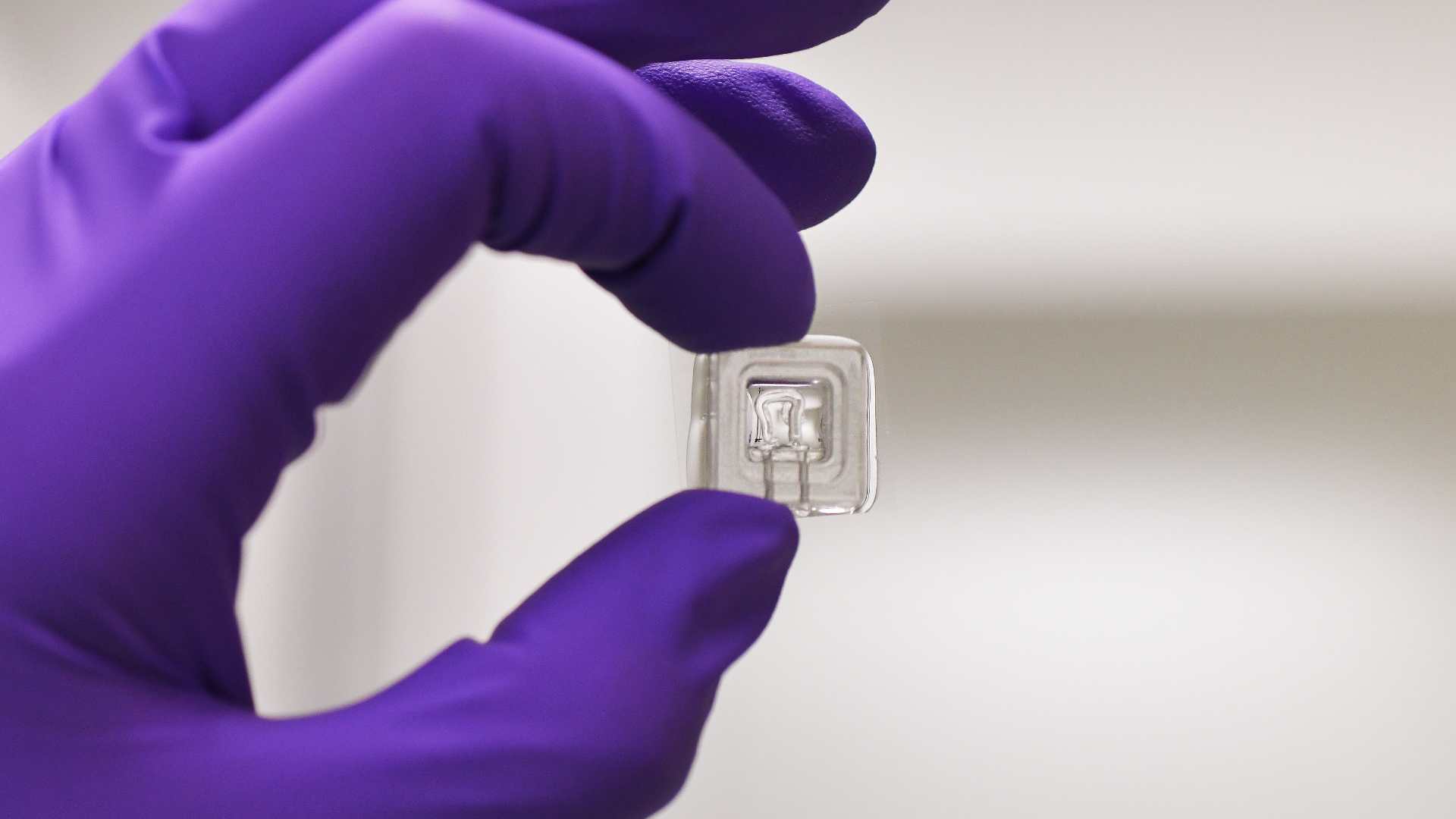
The MOHICAN project aims to develop new medical devices enabling functional testing of patient immune cells as part of routine hospital practice. These innovative devices will integrate microfluidic components coupled with microprinted molecular patterns to recreate structures that mimic secondary and tertiary lymphoid tissues.
Coordinator

Research Director
CNRS
Institutions and establishments involved
Work Packages
CNRS; Inserm; AMU; Université de Bordeaux
Les objectifs scientifiques sont structurés autour de Work Packages :
- WP1 : Développement et production de microdispositifs lymphoïdes avancés
- WP2 : Du laboratoire à la clinique : intégration des essais sur microdispositifs dans le parcours d’immunothérapie personnalisée
Research Program
The objective is to dynamically characterize interactions between effector immune cells, target cells (notably tumor cells), and the immune
microenvironment. Although functional immunological assays are widely used in basic research, their complexity, slowness, and cost hinder their clinical integration. MOHICAN aims to overcome these limitations through a recently patented concept, leveraging the microtechnology expertise of partner laboratories at IINS and LAI. These multifunctional microsystems, based on subcellular-scale micropatterning, will enable fine-tuned modulation of the microenvironment, sorting, identification and positioning of cells, the delivery of spatially and temporally controlled activation signals, and the detection of cell surface markers and cytokine secretion. Measurements will be conducted at the single-cell level, in real time, providing multiparametric kinetic data essential to understanding individual immune responses.
The project will contribute to the development of innovative personalized immunotherapy strategies for hematologic cancers, in collaboration with clinicians from AP-HM (Assistance Publique–Hôpitaux de Marseille) and the Paoli-Calmettes Institute (regional cancer center). To this end, we will develop microdevices tailored to the specific functions of T lymphocytes, CAR-T cells, and NK cells. These systems will be tested in the context of monoclonal antibody therapies (for patient stratification and treatment decision support) and
CAR-T cell therapies (for identifying predictive functional signatures during CAR-T cell preparation, testing cell products in production line before administration, and for post-treatment monitoring). The clinical research protocols (CPP) required for these studies are already in place, along with biobanks containing patient samples accompanied by detailed clinical data and longitudinal follow-up. In parallel, the microchip fabrication process will be optimized and fully automated to ensure rigorous standardization and high-throughput production. A complete technological pipeline—from blood sampling to multiparametric readout of immune functions—will be implemented to enable the integration of this system into routine hospital workflows, addressing current limitations related to technical staff availability.

Expected outcomes
The project is expected to lead to both major scientific advances and medical progress, with a significant impact in the field of immunotherapy monitoring and personalized medicine, in line with the objectives of the PEPR MED-OOC program.
Medical outcomes – Development of routine hospital tools for:
- Stratifying patients eligible for monoclonal antibody therapies;
- Selecting optimal therapeutic options based on the patient’s individual immune response;
- Pre-screening patient-derived cells for personalized cell therapies such as CAR-T cells;
- Monitoring treatment efficacy and safety, including early detection of cytokine release syndrome, B-cell lymphopenia, or relapse. This tool could thus become a central companion diagnostic device for immunotherapy in the hospital setting.
Standardizing functional assays compatible with multicenter clinical trials and hospital environments.
Scientific outcomes
- Providing a platform to study functional heterogeneity at the single-cell level;
- Significantly advancing automated and reproducible micropatterning workflows, both in open-well formats and within microfluidic channels— a key challenge for enabling functional assays ranging from high-throughput screening to perfused dynamic systems — and establishing a new standard in pattern fidelity and assay reproducibility.
The consortium
The MOHICAN consortium brings together leading biophysics teams (LAI – Marseille and IINS – Bordeaux), clinical partners from AP-HM and IPC, as well as the TAGC genomics laboratory. These partners share a strong track record of collaboration, illustrated by joint publications, co-supervision of PhD students, and translational projects. Weekly and bi-monthly coordination meetings are already in place. Technical development will be conducted jointly and progressively transferred to the hospital environment. Intellectual property will be co-managed by Inserm, CNRS, AMU, IPC, and AP-HM under a formal consortium agreement.
CNRS / Inserm / AMU / Laboratory Adhesion and Inflammation – LAI / Theories and Approaches of Genomic Complexity – TAGC / Centre de thérapies cellulaire / Centre d’immunophénomique; Université de Bordeaux / Institut Interdisciplinaire de neurosciences – IINS;
Plus de projets