NeMOCHIP
NeMOCHIP aims to fully harness human pluripotent stem cell (hPSC)-based strategies, advanced bioengineering, and computational modeling to create standardized, patient-derived neuromuscular platforms. By integrating organoids-on-chip and clinical twin models, the project will accelerate neuromuscular disease (NMD) research, enhance precision diagnostic, and reduce reliance on animal models.
Coordinator

Research Director
Inserm
Institutions and establishments involved
Work Packages
CNRS; Inserm; Aix Marseille Université; Sorbonne Université; Université Evry; Université Claude Bernard Lyon 1
Three work packages structure the project:
- WP1 : Engineering Standardized hPSC-Based Neuromuscular Junction-on-Chip Systems: Protocol standardization, optimization, reproducibility, and robust workflows for generating high-fidelity neuromuscular tissues.
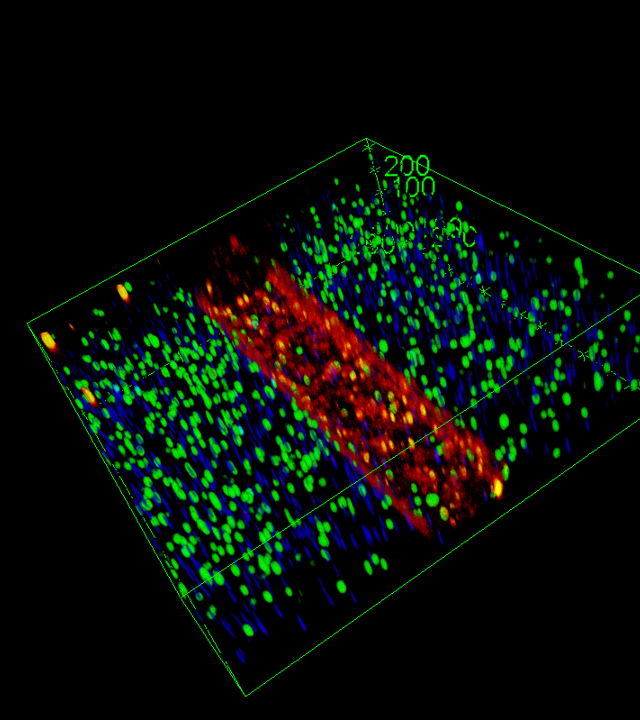
- WP2 : Multi-modal Profiling and integration in a virtual replica: Generation of a comprehensive NMJ atlas combining bulk and single-nucleus RNA-seq, spatial
transcriptomics, imaging, and biosensor data, integrated into a virtual replica. - WP3 : Translating NeMOCHIP Innovations: Development of functional diagnostics and disease models for key NMDs such as congenital myasthenic syndromes (MS), myotonic dystrophy type 1 (DM1), and facioscapulohumeral dystrophy (FSHD), alongside tools for therapeutic screening and patient stratification.
Research program
NMDs are primarily genetic disorders that affect millions worldwide and are often severe, progressive, and currently incurable. Despite recent therapeutic advances, the majority of NMDs lack effective treatments. Diagnostic delays are common, largely driven by restricted access to patient-derived tissues and the limited relevance of animal models, which do not adequately reflect human-specific characteristics of the neuromuscular system, especially at the neuromuscular junction (NMJ), the critical site of nerve-muscle communication.
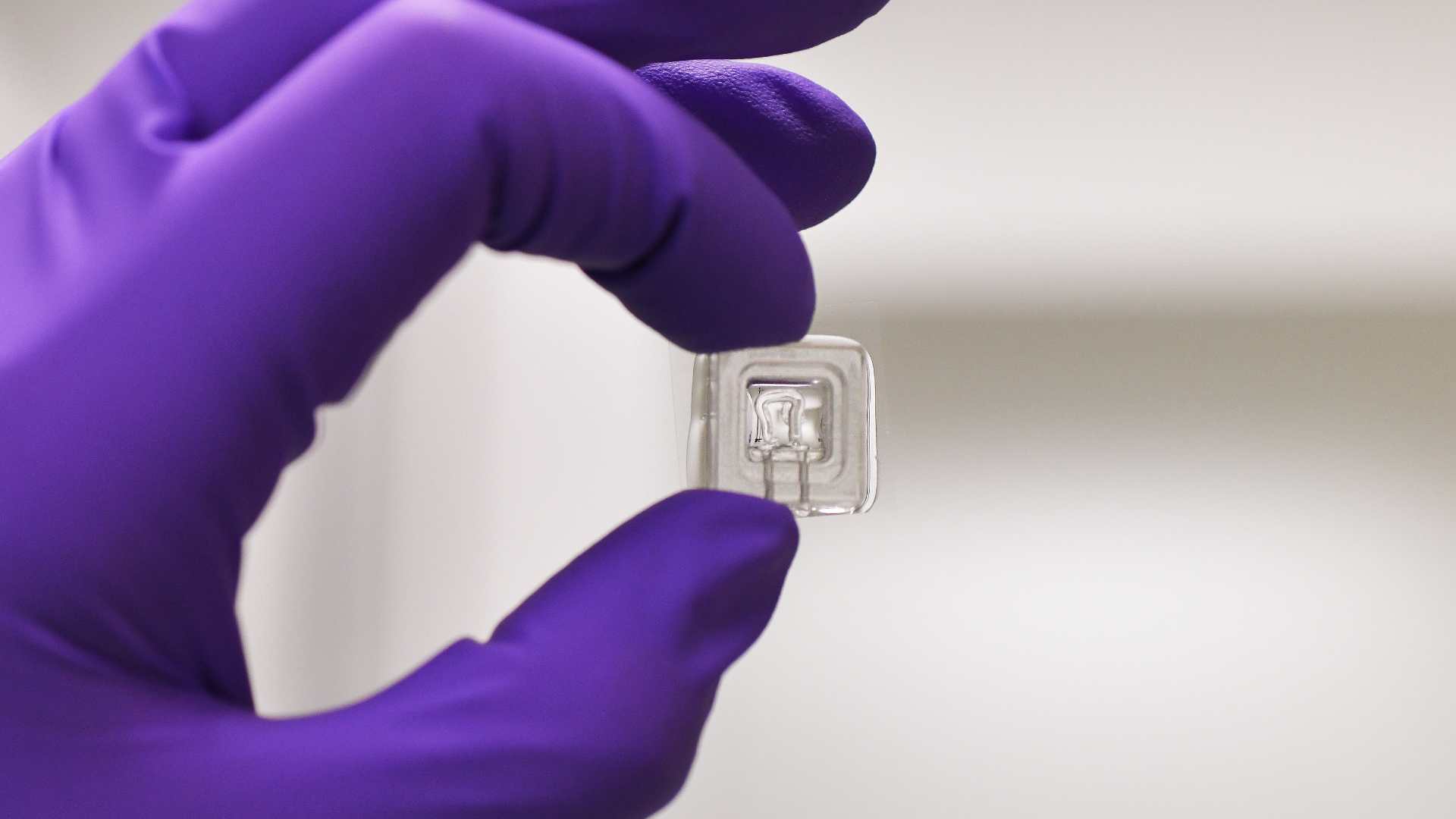
NeMOCHIP addresses these challenges through a comprehensive strategy to develop scalable and harmonized hPSC-derived neuromuscular models. These platforms will incorporate spinal motor neurons, skeletal muscle, and self-organizing NMJs within organ-on-chip systems that simulate physiological microenvironments and enable real-time, non-invasive functional assessment. The project will enforce rigorous quality controls and quantifiable performance metrics to ensure reproducibility across labs and compatibility with clinical and regulatory standards.

Expected outcomes
NeMOCHIP will deliver high-impact translational outcomes including a standardized NMJ-on-chip assay for myasthenic syndromes, disease-specific organoid clinical twin models, and a virtual replica enabling personalized diagnostics and treatment planning. All generated resources, cell lines, atlases, analytical tools, will be made openly accessible to the scientific and clinical communities.
The project’s translational impact extends beyond rare diseases: neuromuscular function is central to many conditions influenced by aging, lifestyle, and chronic illness. NeMOCHIP’s models will also support broader applications in toxicology, regenerative medicine, and drug safety testing.
The consortium
Led by academic, clinical, and industrial teams from Paris, Lyon, Marseille, and Corbeil-Essonnes, NeMOCHIP fosters strong national collaborations and aligns with the goals of the PEPR MED-OoC program by introducing a novel neuromuscular component, currently
underrepresented in the organ-on-chip landscape. It complements ongoing PEPR initiatives, such as “Digital Health” and “Biotherapies & Bioproduction”, through integration of spatial omics, digital modeling, and patient-centered design. NeMOCHIP is also aligned with the French National Plan for Rare Diseases (PNMR4), supporting improved diagnosis and therapy development.
I-Stem; Marseille Medical Genetics; Institut de Myologie; Physiopathologie et générique du neurone et du muscle
Plus de projets