HITOC
HITOC addresses a major limitation in current in vitro models of the human intestine: the lack of physiologically segmented, neurocompetent systems that reflect the structural, functional, and electrophysiological complexity of the gut. While organoid and organ-on-chip (OoC) technologies have revolutionized our ability to model human tissues, existing platforms remain largely restricted to single intestinal segments and are often devoid of key tissue compartments such as the enteric nervous system (ENS). This gap critically hampers our ability to study spatially resolved host-pathogen interactions, microbiota-driven cues, and gut-brain communication, which are inherently segment- and neuron-dependent.
Coordinator

Assistant Professor
Inserm
Institutions and establishments involved
Work Packages
CNRS; Inserm; Aix Marseille Université; Institut Pasteur de Lille; Institut Pasteur de Paris
The scientific objectives are addressed through four work packages:
- WP1 : standardization of hiPSC protocols and data analysis for open science.
- WP2 : developmentally inspired gut on chip mimicking specific intestinal segment.
- WP3 : functional integration and monitoring of the intestinal epithelium and neurons using transparent 3D MEAs.
- WP4 : validation and translational applications of HITOC models.
Research program
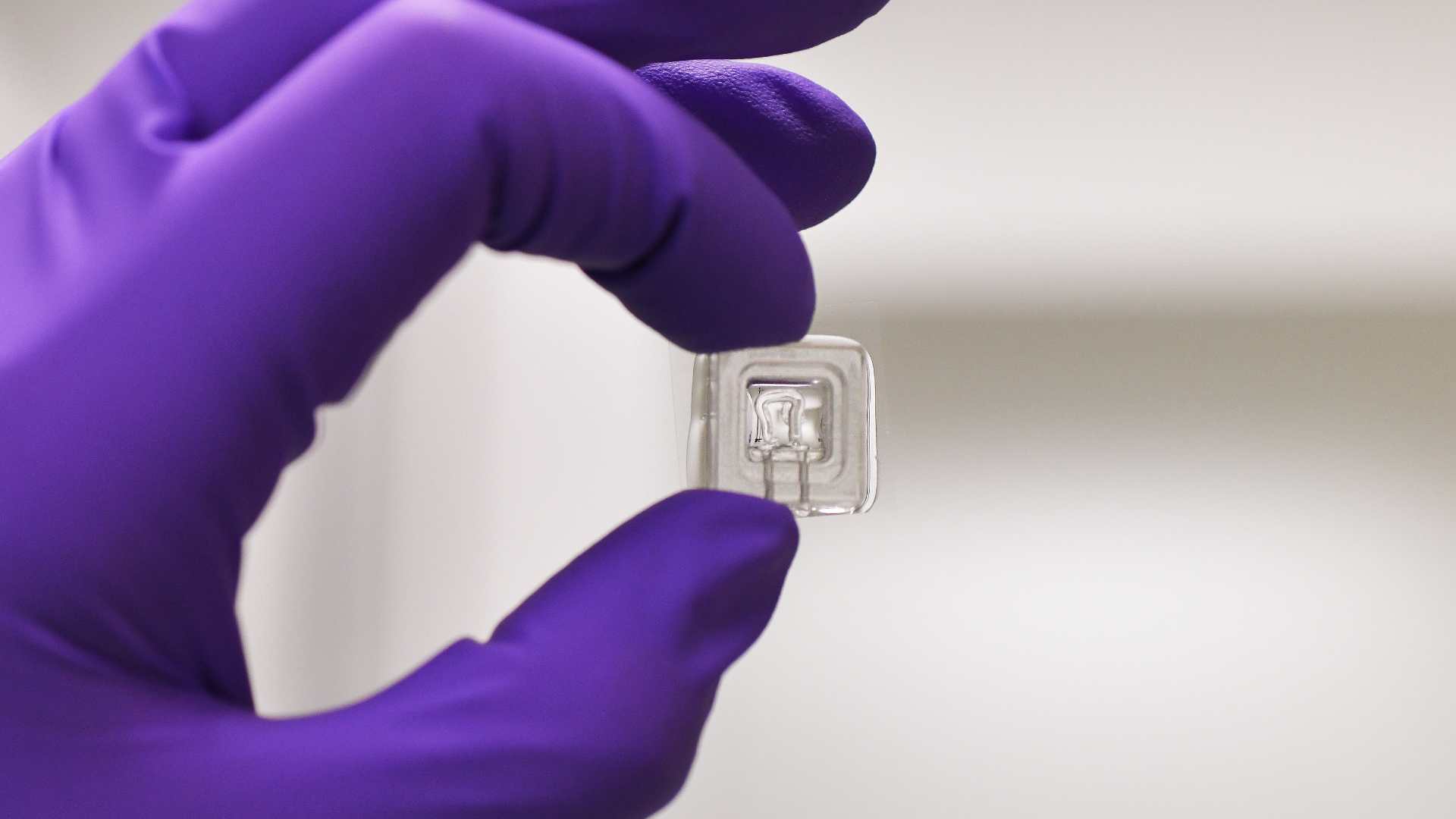
To address this challenge, HITOC proposes a next-generation intestinal tract-on-chip platform derived from human induced pluripotent stem cells (hiPSCs), engineered to recapitulate the rostrocaudal segmentation of the gut (duodenum, jejunum, ileum, colon) and its functional integration with enteric neurons. Our approach is inspired by developmental biology, using spatially controlled morphogen gradients and microstructured confinement to guide segment-specific differentiation on chip.
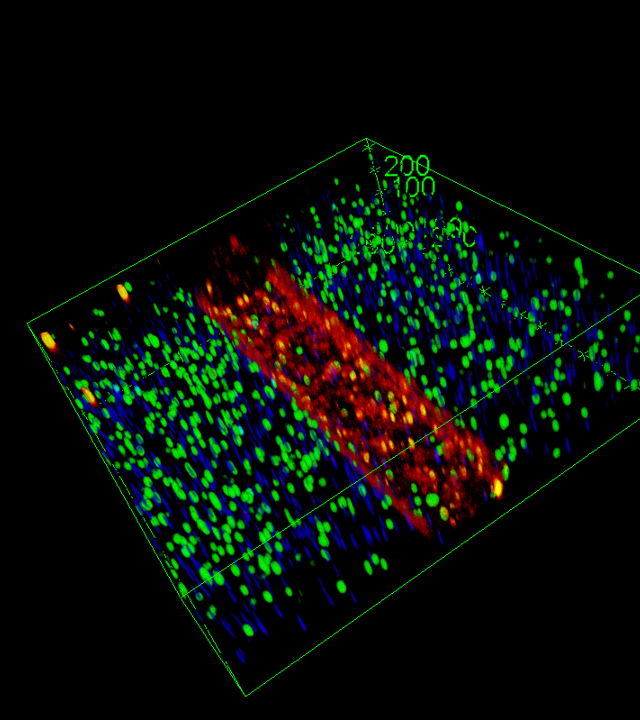
A defining innovation of HITOC lies in the incorporation of a functional ENS component derived from vagal neural crest cells or ENS progenitors, integrated during tissue maturation. This neuro-epithelial interface will be monitored in real time through embedded transparent 3D microelectrode arrays (MEAs), enabling simultaneous electrophysiological recording of ENS activity, calcium imaging, and impedance-based monitoring of epithelial barrier function (via ECIS). These multimodal, label-free biosensors provide unprecedented access to gut physiological function at high temporal and spatial resolution.
As a proof of concept, we will use the HITOC platform to study how human-restricted pathogens (Shigella, Norovirus), known to preferentially target distinct gut regions, alter epithelial integrity and ENS function. In parallel, we will explore the impact of microbiota-derived metabolites—specifically short-chain fatty acids (SCFAs)—on gut neuronal circuits and ENS activity, offering mechanistic insights into gut-brain axis communication.

Expected outcomes
By bridging organoid self-organization with microfabrication and bioelectronic monitoring, HITOC sets a new benchmark for human-relevant intestinal models and will serve as a foundational tool for studying infection, microbiota-host interactions, and neurogastrointestinal disorders in a fully human, preclinical context
The consortium
HITOC brings together a multidisciplinary French consortium with complementary expertise in hiPSC biology, ENS development, microfluidic bioengineering, electrophysiology, and infection biology. This includes teams from Inserm, CNRS, the Institut Pasteur and the Institut Pasteur de Lille, with a strong track record of collaboration and technological innovation. The platform will be modular, interoperable, and openly standardized to ensure broad accessibility and alignment with the PEPR MED-OoC initiative.
CIIL ; IEMN ; IBDM ; TENS ; Plate-Forme Technologique Biomatériaux et Microfluidiques
Plus de projets