MICROCOSM
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, characterized by progressive airflow limitation and frequent exacerbations that worsen patient outcomes. While biologics have revolutionized asthma treatment, their application in COPD has been challenging due to patient heterogeneity and low clinical trial success rates. The recent approval by the Food and Drug Administration (FDA) of dupilumab for eosinophilic COPD represents a breakthrough, but its benefits are limited to a subset of patients.
Coordinator

Professor
Université de Bordeaux
Institutions and establishments involved
Work Packages
Inserm; Université de Bordeaux; Université Paris Est Créteil; CEA; CHU de Bordeaux; APHP
This study addresses this challenge through three interconnected work packages (WPs):
- WP1: Generation and cryopreservation of progenitor cells (lung and skeletal muscle) to
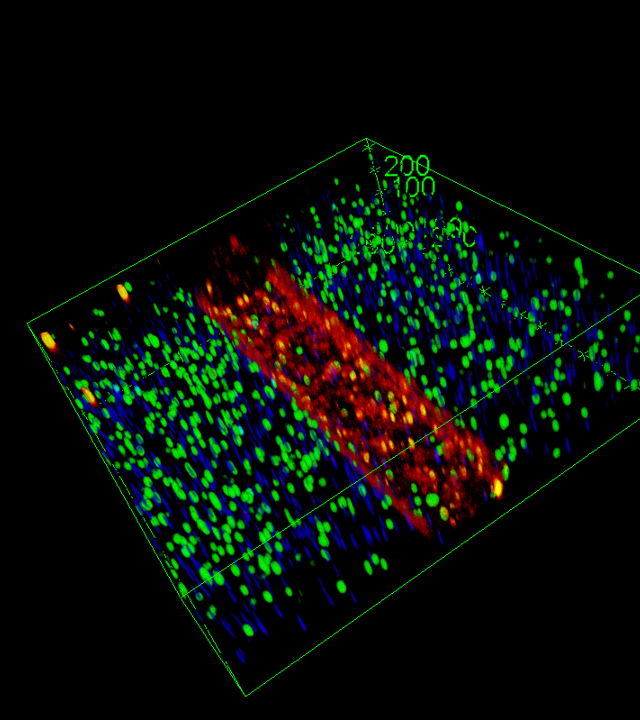
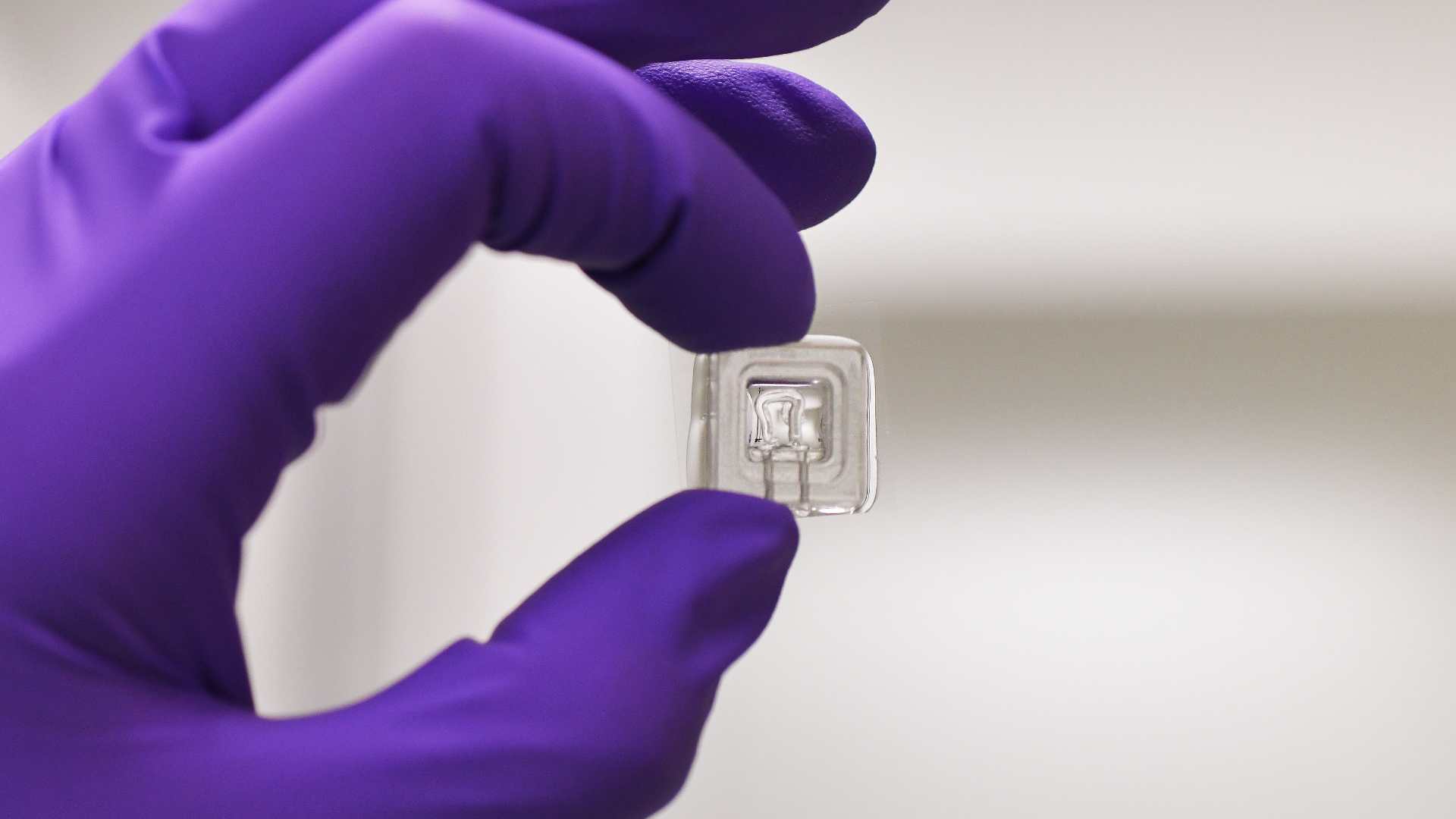
create patient-relevant in vitro models. - WP2: Development of a multi-organ-on-chip platform that integrates lung (airway and
alveolar) and muscle cells to mimic COPD pathophysiology. - WP3: Prediction of patient responsiveness to biologics and selection of the most effective
biologics for personalized biotherapy in COPD.
Research program
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, characterized by progressive airflow limitation and frequent exacerbations that worsen patient outcomes. While biologics have revolutionized asthma treatment, their application in COPD has been challenging due to patient heterogeneity and low clinical trial success rates. The recent approval by the Food and Drug Administration (FDA) of dupilumab for eosinophilic COPD represents a breakthrough, but its benefits are limited to a subset of patients.
Emerging therapies targeting epithelial-derived alarmins, such as TSLP and IL-33, offer a promising avenue for COPD treatment, with several phase II and III trials underway.
However, identifying responders to anti-biologics remains a significant challenge, as no universally validated
biomarker exists. In addition, systemic effects of biologics remain to be determined, such as effects on skeletal muscles. Indeed, sarcopenia further increases mortality risk in COPD, highlighting the need for improved therapeutic strategies, but the lack of predictive models further
hampers personalized treatment approaches. Emerging methods, such as organoids and organ-on-a-chip technologies, offer more human-relevant data and are showing great promise as predictive tools, for new treatments as well as for existing drugs. Our project aims to develop a multi-organ-chip platform to predict COPD patient responses to biologics and choose the most effective biologics.

Expected outcomes
By leveraging advanced in vitro models that better mimic human physiology, this approach could pave the way for personalized immunotherapy in COPD, improving patient outcomes and therapeutic success rates.
The consortium
To achieve this vision, the project brings together a multidisciplinary team that spans pulmonology, lung and skeletal muscle physiology, metabolomics, microfluidics and organ-on-chip technology. The research consortium, including physicians actively involved in patients’
care and experts in lung biology and microphysiological systems, is structured to ensure a close interaction between hospital-based clinical research units and academic laboratories, with access to biobanking, microfluidics platforms, and advanced imaging and analytical tools. This integration of expertise ensures that the scientific strategy is not only ambitious but also feasible, grounded in well-established networks and infrastructures.
Centre de recherche Cardio-Thoracique de Bordeaux; Institut Mondor de Recherche Biomédicale;
Plus de projets